Inside Angle
From 3M Health Information Systems
Seeing pink in February: Documentation specificity with breast studies
Every October we see pink during breast cancer awareness month. However, with the recent 2019 CPT updates that took effect January 1, the addition of some new breast-related codes reminded me how important and prevalent diagnostic breast studies are. The National Breast Cancer Foundation states, “1 in 8 women will be diagnosed with breast cancer in her lifetime. There is currently no known cure for breast cancer, and its early diagnosis is critical to survival.” From a clinical perspective, awareness and being proactive is key; the same can be said from a coding and documentation perspective.
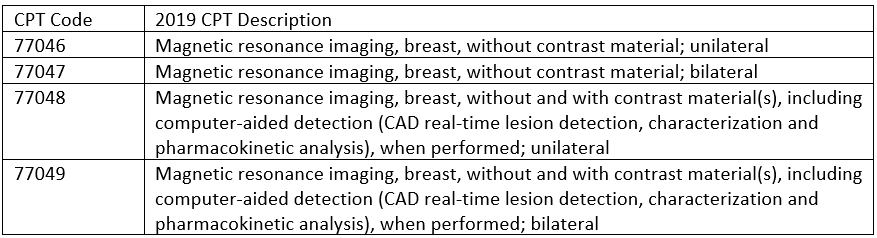
In the last few years we have seen consistent changes to CPT codes. 2018 brought the departure of G codes that CMS previously established for the reporting of mammogram codes. Along with that came changes to tomosynthesis CPT code reporting. Now in 2019, we’ve once again seen CPT changes to breast-related codes. This time with an expansion to the breast MRI code set brought about by establishing formal contrast status for both unilateral and bilateral studies.
2018 CPT Codes
2019 CPT Codes
The establishment of contrast status means it’s imperative for specific documentation to include “without contrast” and “without and with contrast.” Coders should be ready to give this feedback to providers so that documentation is present for appropriate code selection, whether it is directly in the procedure or in the technique. In addition, coders should be aware of what is ordered vs. what is actually being documented. The practice of checking this helps to eliminate assumptive coding and enhances compliance as well as documentation improvement.
Definitive documentation doesn’t stop at procedures. Documentation best practices supporting diagnoses should be reviewed regularly as well. With the increase in many breast exam encounters, including mammogram and breast ultrasound, be sure that for screening-based patient encounters, the specific type of screening being performed or the condition being screened for is established. Z12.31 (Encounter for screening mammogram for malignant neoplasm of breast) is reported for screening mammograms while Z12.39 (Encounter for other screening for malignant neoplasm of breast) has been established for reporting screening studies for breast cancer outside the scope of mammograms.
Another important component concerns documentation around abnormal findings. Over the years we have seen more specificity in diagnosis reporting for breast conditions. We had historically seen findings such as lumps reported based on laterality. This has changed most recently with the N63.XX series of ICD-10 codes which in the last few years has included quadrant reporting to provide a more precise location of the identified lumps.
The changes highlighted the need to not only include laterality, but location of the lump whether in a breast quadrant, axillary tail, or subareolar region. Be aware of how specific requirements impact your documentation and what that means for your practice. Will upper or lower and outer or inner quadrant location be the basis for reporting the quadrant, or will o’clock documentation be an option? Know how your providers document and be sure to provide feedback if that language is missing.
Encourage proactive habits, provide feedback for missing or limited documentation, and make sure all elements of the exam itself are being clearly documented and support code selection. Take a cue from the breast health awareness movement and remember to stay vigilant when it comes to coding and documentation of breast studies.
Allison Morgan is a clinical development analyst at 3M Health Information Systems.