Inside Angle
From 3M Health Information Systems
Eyes on Eylea and Lucentis
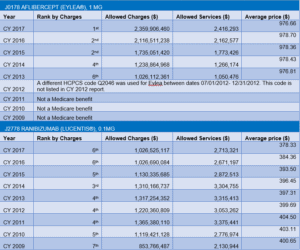
Recently, I came across an OIG announcement “Review of Medicare Part B Claims for Intravitreal Injections of Eylea and Lucentis” released in June 2019. Upon reading this announcement, I pulled the latest CY 2017 Top 200 Level II HCPCS Codes Ranked by Charges for Part B Medicare. This report lists Eylea (HCPCS code J0178) as the topmost utilized drug and Lucentis (HCPCS code J2778) as the sixth most utilized drug for 2017, contributing to a Medicare expenditure of nearly 2.36 billion and 1.03 billion dollars respectively. In fact, upon reviewing other years’ available reports, these two drugs have been among the highest cost year after year.
Chart 1 is a year by year utilization chart that lists these two drugs with their rank by charges, allowed charges, allowed services and average price (created from available reports at part B utilization reports). Besides being among the highest in their yearly utilization, also notice the following:
- The higher cost (average price) for Eylea as compared to Lucentis.
- A fast increase in services for Eylea year after year.
- A gradual drop in Lucentis services since CY 2014.
Chart 1: Yearly utilization for Eylea and Lucentis taken from part B utilization reports
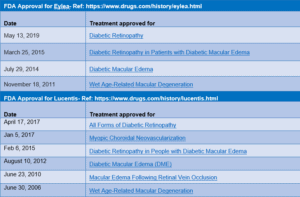
So, what are Eylea and Lucentis indicated for? Why are they contributing so significantly to Medicare spending?—Eylea and Lucentis are anti-vascular endothelial growth factor (anti-VEGF) drugs injected in the eye intravitreally to treat conditions such as wet age-related macular degeneration (AMD), a leading cause of blindness among the Medicare population. These are groundbreaking treatments approved by FDA just over the past few years. See Chart 2 below for FDA approval of these drugs for different conditions by date. As you will see, they are mostly indicated for same conditions, but Eylea was approved later than Lucentis. Could increase in services for Eylea over the years (noticed in Chart 1) be related to increase in number of indications approved over that time? Or, are doctors gradually shifting from Lucentis to Eylea?
Chart 2: FDA approval dates and indications for Eylea and Lucentis
In considering the current OIG announcement, what are the Medicare requirements related to Eylea and Lucentis? Here’s a related excerpt from the OIG published report:
“We will review claims for intravitreal injections of Eylea and/or Lucentis and the other services billed on the same day as the injection, including evaluation and management services, to determine whether the services were reasonable and necessary and met Medicare requirements.”
This leads to the following questions:statement:
- How should reasonable and necessary services for these drugs be determined?
- What other services can be billed the same day?
- Are evaluation and management services on the same day covered?
- Are there any other requirements?
Most answers to these questions can be found in Local Coverage Determinations (LCDs) and/or associated articles. Currently, only four out of seven Part B Medicare Administrative Contractors (MACs) have published guidance for these drugs.
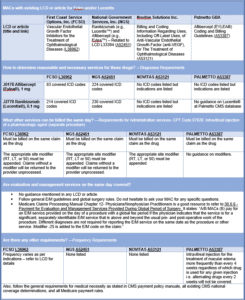
Next, let’s look at different MAC requirements related to these drugs. Here’s Chart 3 I created to understand requirements from each of the four MACs with LCD or article in place.
[Disclaimer: This chart is in no way an evaluation of any existing LCDs. It also should not be used as replacement for existing Medicare guidance. Please check original sources applicable as per date of service for details on each requirement.]
Chart 3: Requirements listed by MACs for Eylea and Lucentis
Chart 3 shows that some guidelines vary from MAC to MAC. Out of these four MACs, two MACs—First Coast Service Options (FCSO) and National Government Services (NGS)—have listed covered ICD-10 codes. However, I’m concerned about the differences in diagnoses between these two MACs. For example, FCSO has 83 covered ICDs for Eylea whereas NGS has 214 covered ICDs! NGS lists additional diagnoses such as Diabetic retinopathy without macular edema, Diabetic retinopathy with traction retinal detachment, Central retinal vein occlusion with retinal neovascularization and stable type, Cystoid macular degeneration, and Retinal edema. Does this mean Eylea could be a non-covered service if a Medicare beneficiary diagnosed with Diabetic retinopathy without macular edema was residing in Florida (claims processed by First Coast)?
To prevent medical necessity denials, it’s important that providers follow their MAC guidelines up front rather than correcting the rejected claims and appealing the denied claims after the fact. If there is disagreement with an existing LCD or when additional services are needed, MACs should be contacted for clarification and for a reconsideration request.
I also want to emphasize that absence of an LCD or article in other jurisdictions does not mean the claims will not be reviewed. Claims may be monitored through post-payment data analysis and subsequent medical review audits. In general, providers should follow FDA-approved indications, dosage and frequency standards. In the absence of an LCD or article, it is a wise practice to check if there are any additional guidelines posted at MACs’ individual websites, to contact them for pre-approval and to always document medical necessity for all services.
Based on these observations, I’m eagerly waiting to see what will come of the OIG findings, if any claims were incorrectly paid, if some were unreasonable and unnecessary and what recommendations will be made to Medicare and its contractors.
[NOTE: Links and data in this blog are as of July 24, 2019 from the Medicare Coverage Database and are subject to change.]
Divya Verma, is a compliance analyst for the Medical Necessity and Compliance division within 3M Health Information Systems.
Providers: Get the help you need to comply with these complex Medicare requirements.